|
|
Institution and research group
Authors: Mika M Sampo, MD1,2, Maija Tarkkanen, PhD2, Erkki J Tukiainen, PhD3, Pentscho
Popov, PhD3, Mikael Lundin, MD6, Carl P Blomqvist, PhD2, Pelle Gustafsson, PhD4,
Tom O Böhling, PhD1, Johan Lundin, MD, PhD2,5,6
Affiliations: Department of Pathology, HUSLAB and University of Helsinki,
Finland, PO Box 400, the Hospital District of Helsinki and Uusimaa1, Department of Oncology, Helsinki
University Central Hospital (HUCH), Finland PO Box 180, the Hospital District of Helsinki and Uusimaa2,
Department of Plastic Surgery, HUCH, Finland, PO Box 288, the Hospital District of Helsinki and Uusimaa3,
Department of Orthopedics, Lund University, Sweden, Lund University Hospital
SE-221 85 Lund, Sweden4, Division of Global Health, Karolinska
Institutet, Stockholm, Sweden5, Institute for Molecular Medicine
Finland, University of Helsinki, Helsinki, Finland, PO Box 20, 00014 Helsinki
University6
|
Data set and variables
All patients referred for non-metastatic, primary or recurrent soft tissue
sarcoma (STS) of the extremities
or trunk wall to the Soft Tissue Sarcoma Group between August 1987 and December
2002 are included. Exclusion criteria comprised: extraskeletal osteosarcoma, chondrosarcoma,
Ewing/PNET family tumour, angiosarcoma, alveolar soft tissue sarcoma, epitheloid
sarcoma, clear cell sarcoma, atypical lipoma/grade I liposarcoma, dermatofibrosarcoma
protuberans, preoperative radiotherapy or chemotherapy or both or postoperative
chemotherapy (n=15). In 84 cases we were unable to retrieve the original histological
slides leaving 294 cases for analysis.
Necrosis and vascular invasion were reported as defined by Gustafson and colleagues.1
They were re-assessed for the present study and classified as absent or present.
Tumour size (cm) was recorded as the largest diameter of tumour in the surgical
specimen reported by the original pathologist. The pathologist assigned the histological
malignancy grade of the tumour based on a four-tiered grading scale modified from
Broders and colleagues.2-3 Grades 1 and 2 are low and grades 3 and 4 are high grades.
Subcutaneous tumours with or without cutaneous extension but without involvement
of the deep fascia were defined superficial, all others deep.
References:
1. Gustafson, P, Akerman, M, Alvegard, TA et al. Prognostic information in soft
tissue sarcoma using tumour size, vascular invasion and microscopic tumour necrosis-the
SIN-system. European journal of cancer 2003; 39: 1568-76.
2. Broders, AC, Meyerding, HW. Pathological features of soft tissue sarcoma. Surg
Gynecol Obstet 1939; 69: 267-80.
3. Angervall, L, Kindblom, LG, Rydholm, A, Stener, B. The diagnosis and prognosis
of soft tissue tumors. Seminars in diagnostic pathology 1986; 3: 240-58.
|
Statistics and system tools
Sarcoma-specific survival (SSS) was calculated from the date of the diagnosis to
death from sarcoma. Deaths due to other causes than sarcoma were censored. A Cox
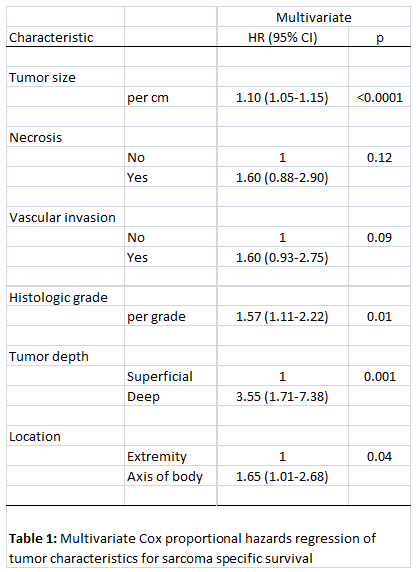
proportional hazards model was fitted entering the following covariates: tumour
size in millimeters (continuous), necrosis (absent vs present), vascular invasion
(absent vs present), tumour depth (superficial vs deep), location (extremity vs
axis of body), and histologic grade (four levels). Please see Table 1 for the final
regression model.
|
|
Based on the fitted Cox regression models 10-year sarcoma-specific survival is estimated.
With the β-coefficients of a Cox model a prognostic index (PI) is estimated. The
survival curve corresponding to the average PI value is computed, which is similar
to the average survival for the total group. If the average PI value is taken as
baseline reference, the relative risk (RR) of an individual patient is given by
RR = eestimated PI/eaverage PI = eestimatedPI-average PI
With the average survival curve corresponding to the average PI value and the patient's
relative risk, an expected survival curve for that patient can be computed. For
example, in the current series the cumulative 10-year survival is 0.71, and the
expected 10-year survival for a patient with the relative risk of RR is 0.71RR.
|
Disclosure and sources of funding
The funding source had no role in the study design, collection, analysis, interpretation
of data, or writing of the report.
The project has been funded exclusively by competed
research funds. The major supporters are governmental EVO funds of the Helsinki
University Central Hospital, the Finnish Cancer Society, the Sigrid Juselius Foundation,
the K. Albin Johansson Foundation, Finska Läkaresällskapet and Duodecim Foundation.
These financial contributors are warmly acknowledged.
|
|
|
|